Congratulations to Kyle Novakowski for receiving an OGS scholarship for his PhD research on macrophage phagocytic receptors. Way to go Kyle!
Tag Archives: macrophage
New additions to the “Protocols” section
We’ve added some major new protocols to the protocol section. We know have a section on basic molecular biology, some protocols for 16s rRNA sequencing, some updates to our transfection and NF-kB reporter protocols, etc. Take a gander & enjoy.
Extra! Extra! Bowdish Gang Uncovers Truth About IL-17 In The Nose!
The nose is the gateway to the soul… or the lungs at least… making it an important point of first contact between our fragile bodies and the hordes of superbugs attempting to take over the world. Only the brave immunologist has the power to save us from this dire threat. While it’s been known for a few years now that the inflammatory cytokine IL-17A is key to the control of many respiratory infections, no-one has been able to provide any information on the source of this cytokine in nasal infections or how this production is regulated. No more!
Post-doctoral fellow Chris Verschoor and Ph.D. Candidate Mike Dorrington, both trainees in Dr. Dawn Bowdish’s lab have recently had their manuscript “MicroRNA-155 is required for the clearance of Streptococcus pneumoniae from the nasopharynx” accepted for publication in Infection & Immunity. The paper, which was produced in collaboration with Dr. Param Nair of the Firestone Institute, outlines how microRNA- (miR-)155 regulates the immune response to S. pneumoniae colonization in the nasal passages of mice by stimulating the differentiation of Th17 cells. These cells then produce large amounts of IL-17A, which then acts as a chemotactic agent for macrophages, which have awesome swords and stuff that kill the bacteria and save the world! (macrophages are the best cells, by the way)
This paper is the first to show a direct connection between IL-17A-producing T cells and the clearance of a bacterial pathogen from the nasopharynx. It is also the first to show a phenotype of IL-17A deficiency without completely knocking out the cytokine itself. It represents a significant step forward in understanding the regulation of intranasal immune responses to bacterial colonization and how innate and adaptive immune networks collaborate in clearing these events. Way to go Bowdish Lab!
For more information please visit www.bowdish.ca/lab and check out the paper in an upcoming edition of Infection & Immunity.
Kaiser JC, Verschoor CP, Surette MG, Bowdish DME. Host cytokine responses distinguish invasive from airway isolates of the Streptococcus milleri/anginosis group. BMC Infect Dis. 2014 Sep 11;14:498. doi: 10.1186/1471-2334-14-498.
Kaiser JC, Verschoor CP, Surette MG, Bowdish DME. Host cytokine responses distinguish invasive from airway isolates of the Streptococcus milleri/anginosis group. BMC Infect Dis. 2014 Sep 11;14:498. doi: 10.1186/1471-2334-14-498.
This paper demonstrates that there are host- and strain- specific responses to isolates of the Streptococcus milleri/anginosis group and that isolates from invasive disease appear to be more immunostimulatory than those from commensal relationships.
“Comprehensive & simultaneous coverage of lipid and polar metabolites for endogenous cellular metabolomics using HILIC-TOF-MS” 2014. Fei et al. Anal Bioanal Chem.
The Bowdish lab enters a collaborative agreement with Qu Biologics.
The lab of Dr. Dawn Bowdish at the McMaster University Immunology Research Centre (MIRC) has recently begun collaboration with the Vancouver-based pharmaceutical company Qu Biologics on preclinical studies investigating the role of macrophage dysfunction in chronic inflammation.
Qu Biologics has developed Site Specific Immunomodulators (SSIs), which aim to “reboot” the body’s innate immune system in targeted organs or tissues to reverse chronic inflammation.
“Macrophages are important cells of the innate immune system. There is growing evidence that macrophage dysfunction underlies many important common chronic diseases, including cancer and autoimmune disease,” said Dr. Hal Gunn, CEO of Qu Biologics. “This collaboration will be invaluable to assist in our understanding of the benefits of SSI therapy on macrophage function as it relates to chronic inflammation and immune dysfunction.” Dr. Gunn added.
The studies will test whether a lung-specific SSI therapy can restore normal lung and bone marrow-derived macrophage function using a variety of in vivo and in vitro assays.
Dr. Bowdish adds “This is an ambitious and exciting project that takes a fundamentally different approach to tackling the problem of chronic inflammation, which has been very resistant to therapeutic intervention. My team is thrilled to be working together on a problem that affects the lives of so many Canadians.” This work capitalizes on the resources and immunology expertise of the McMaster Immunology Research Centre and Dr. Bowdish’s research interests in how inflammation impairs macrophage function.
About Qu Biologics
Qu Biologics develops Site Specific Immunomodulators (SSI), a novel class of immunotherapies that aim to reboot the body’s immune system. SSIs are designed to stimulate an immune response in targeted organs or tissues to potentially reverse the chronic inflammation underlying many conditions including cancer and autoimmune disease. The company recently launched a Phase 1/2 clinical trial to research SSI therapy for the treatment of Crohn’s disease.
Backed by a prestigious group of scientific advisors and board members, Qu Biologics is led by a management team that includes co-founder and CEO Dr. Hal Gunn, a physician and expert on the body’s immune response to chronic disease; and Chief Medical Officer Dr. Simon Sutcliffe, former CEO of the BC Cancer Agency and a distinguished clinician, scientist and leader in cancer control in Canada and internationally. For more information, visit www.qubiologics.com and www.qucrohnstrial.com.
For more details and to see the original press release here:
Collaborator update: Fan Fei wins “Glasgow Polyomics & University of Strathclyde Young Scientist Award” at the 9th Annual Conference of the Metabolomics Society!
Fan Fei (PhD candidate), under the supervision of Dr. Brian McCarry, and in conjunction with Bowdish lab undergraduate Keith Lee, studies age related changes in the inflammatory response from a metabolomics perspective. Funded by the Russell Bell Travel Scholarship award, she attended the 9th Annual conference of the Metabolomics Society. July 1-4, 2013, SECC Glasgow. She won the “Glasgow Polyomics & University of Strathclyde Young Scientist Award” for outstanding poster presentation of research in the field of metabolomics at the Metabolomic Conference 2013 in Glasgow Scotland for her work “Comprehensive Metabolomic Analysis Reveals Major Differences in the Macrophage Inflammatory Response Between Young and Aged Mice”. Way to go Fan!
Extra, Extra, Read all about it! The Bowdish lab elucidates the evolution of the class A scavenger receptors.
Congratulations to Fiona Whelan (MSc) for publishing her first manuscript “The Evolution of the class A scavenger receptors” in BMC Evolutionary Biology. The scavenger receptors are an evolutionarily ancient family of proteins required for host defence and homeostasis but teasing apart their function and even their structure has been challenging. The goal of this manuscript was to use evolution as a guide to discover how the class A scavenger receptor family was formed and to identify regions of conservation and hence probable functional importance for future study. Phagocytic receptors such as the class A scavenger receptors are integral members of the innate immune response, which is conserved in all classes of life and after reproduction and nutrient acquisition is probably the major most fundamental requirement for survival.
There are essentially only four basic mechanisms of the innate immune system – agglutination (e.g. lectins), lysis/neutralization (e.g complement, antimicrobial peptides), phagocytosis (e.g. scavenger receptors), and pro-inflammatory signalling (e.g. the toll like receptors). The fact that these processes are ancient and have been so strongly preserved is a testament to their importance. Of these, phagocytosis is likely the most ancient process and was probably adapted from its original purpose of nutrient ingestion . One might hypothesize that phagocytosis was truly the genesis of the immune system since our single celled ancestors had to distinguish between “self” and “non-self” in order to distinguish between food and their own daughter cells. From there phagocytosis became essential to fundamental processes such as embryonic development, pathogen recognition, and homeostatic clearance of senescent cells. Without phagocytosis, the transition to more complicated life forms could not have occurred.
Although there have been excellent evolutionary analyses of the lectins, toll like receptors and complement pathways, very little is known about the evolution of the phagocytic receptors. The class A scavenger receptors are an excellent example of these multifunctional receptors as they are involved in both host defence and homestasis. Since the phagocytic receptors in general and the scavenger receptors in particular are a diverse group of proteins,it has been challenging to understand how members within a group are related. Indeed, the first goal of this manuscript was to definitively demonstrate that the members of the class A scavenger receptors, which had been grouped together based on a ragtag combination of ligand binding and some degree of amino acid similarity, were actually a family at all. Since we were able to trace a probable path of gene duplication and consequent functionalization, we are confident that the 5 members (SRAI/II, MARCO, SCARA3/4/5) are actually related. Interestingly the class A scavenger receptors may have acquired their long stalk like form with a single scavenger receptor cysteine rich domain (SRCR) around the time of the evolution of fish since, although SRCR domain can be found in invertebrates and single celled organisms, we could not find anything that resembled a modern class A scavenger receptor in any genomes of evolutionarily more ancient organisms such as jellyfish, lampreys and insects.
Because elucidating the function of the specific domains of the scavenger receptors has been so challenging (even the function of the SRCR domain is unclear), ultimately we want to use evolution as a guide to which domains are functionally important (i.e. conserved). In this regard we found that there is a common conserved region in the collagenous domain, which in the type member SRAI, is believed to be the ligand binding domain. In addition conserved domains were identified in the cytoplasmic tail and the coiled-coiled domain. Future experiments will be performed to determine if these domains are necessary for structure, expression, cellular localization or phagocytic function.
The best part of our paper is that it is Open Access so you can enjoy reading it in provisional form today at http://www.biomedcentral.com/1471-2148/12/227/abstract. If you have questions, comments or thoughts, please feel free to contact Dr. Dawn Bowdish at bowdish@mcmaster.ca or on her Google+ account. Dr. Bowdish is always interested in talking with undergraduate or graduate students interested in pursuing studies that use bioinformatics to answer questions about the scavenger receptors, phagocytosis, and structure/function relationships.
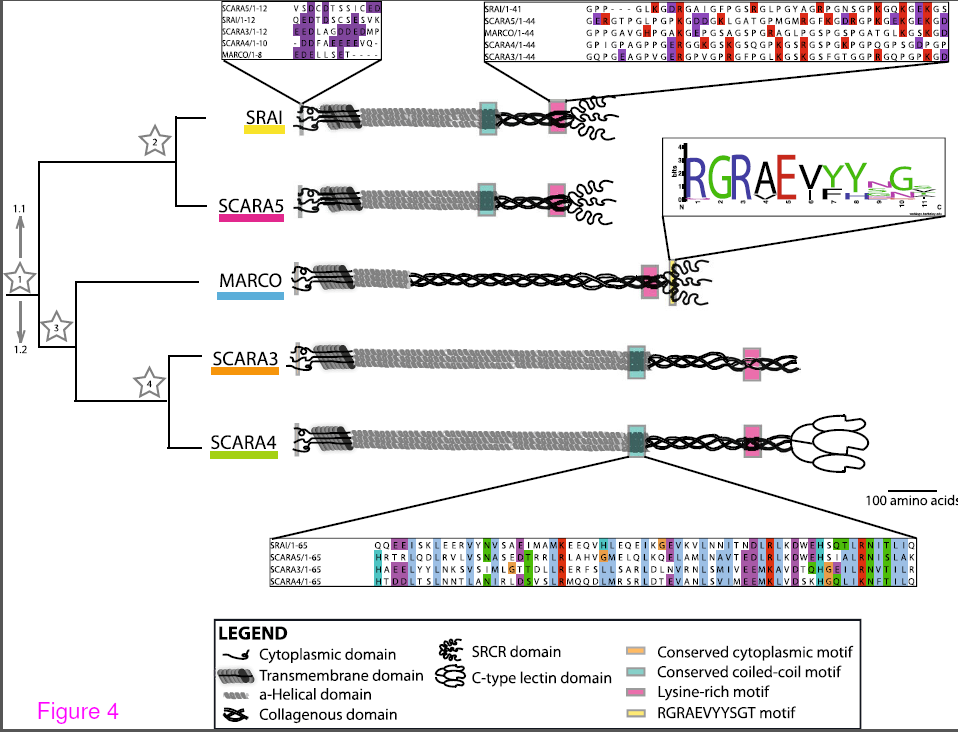
A summary of the common motifs in the class A scavenger receptor protein sequences. Conserved motifs present in the protein sequences of these receptors are indicated with coloured boxes at their approximate position within the protein with shout out boxes used to show the level of conservation across the aligned Homo sapiens sequences. From Whelan et al. BMC Evol Biol. BMC Evolutionary Biology 2012, 12:227
Dr. Bowdish receives a CIHR Operating Grant from the Institute of Aging.
Dr. Bowdish’s grant, titled Macrophage function changes and contributes to susceptibility to infectious disease, was awarded $730,124 from the CIHR Institute of Aging. This new grant will examine age-related changes in monocytes and macrophages to better understand aspects of aging that increase suceptibility to Streptococcus pneumoniae infection. This grant will likely allow Dr. Bowdish to hire a new post-doctoral fellow and graduate student. Interested applicants should consult the FAQ page.
MIRC scientists were highly successful in this recent round of CIHR funding (especially considering the low rates of funding!). To see who else got funded, click here.
Michael Dorrington presents his work on pattern recognition receptors which recognize Streptococcus pneumoniae, November 2, 2011
Mike will be presenting at this week’s “Work in Progress” seminar. For details click kafka and dorrington-nov-2-11.